Chemical degradation is a process through which a compound is deteriorated by employing some external elements. The external components may be other chemicals or ambient conditions, the likes of heat, moisture, or sunlight. But note, there are different types of chemical degradation, one of which is pyrolysis. The pyrolysis process involves the thermal decomposition of compounds at high temperatures in an inert atmosphere. Several plants facilitate the process of pyrolysis, like MoreGreen. In this post, we seek to provide detailed information on the process of pyrolysis.
The pyrolysis Process
A complete pyrolysis process involves six main steps;
Steps in Pyrolysis
1. Pretreatment of raw materials
Different devices can be used in the pretreatment of raw materials. This step of the operation aims to make sure that the raw materials are uniformly processed in the reactor. For a successful pyrolysis process, the following implementations have to be made in this step of the process;
- The raw materials should be sized 50mm or less
- The amount of moisture in the raw materials should not exceed 20%
- The raw materials can be disinfected especially when processing medical wastes
2. Feeding
This is where the raw materials are fed to the machine. Depending on the machine being used in the process, the speed at which the raw materials are fed to the device can be physically adjusted or automatically.
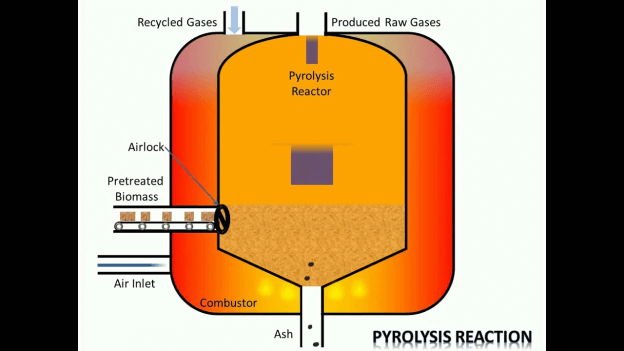
3. Pyrolysis

At this stage, the raw materials are heated above their decomposition temperature, and this results in the chemical bonds being broken in the molecules of the raw materials. The fragments become smaller as the process continues. However, at times, the particles may combine to come up with larger masses. During this process, an element such as oxygen or water may be added to make the process more efficient. But to avoid opposing reactions, the process is done in a vacuum, which also lowers the boiling point of the derivatives hence improving their repossession.
4. Condensation in the condenser system
Some of the end products of pyrolysis are condensable liquids and non-condensable gases. In the case of condensable liquids, in the condenser system, the final product is liquified. After this process, the condensable liquid can be put to use immediately.
5. The Hydroseal
This is a new process that non-condensable gases go through. In this stage, the combustible gas enters the Hydroseal system where the sulfur components are expelled and recycled to be used in the heating process as a reactor. Once sulfur has been removed from the gas, the gas is ready to be discharged.
6. Discharge
Typically, pyrolysis plants connect conveyor belts to the reactors to work as a means of discharging the final products. At this stage, the temperature declined, and the end products (can be charcoal or gas or fuel or steel wire) are collected directly.

Bottom Line
Pyrolysis can be applied to organic products of all kinds, pure products, or mixtures. Different plants use different processes and various machinery (pyrolysis reactors) in the process. However, the end products for all these cases are either charcoal black, steel wire, or fuel oil in either liquid or gas form.